Homework Help - Tumblr Posts
what’s your ‘thing’ as a friend. i don’t mean like ‘oh im the mom friend’ i mean like what’s the Thing where if one of your friends was looking for a specific interaction they’d message you first. personally i can always be relied upon to get hyped about bugs, literature, and cursed internet images.
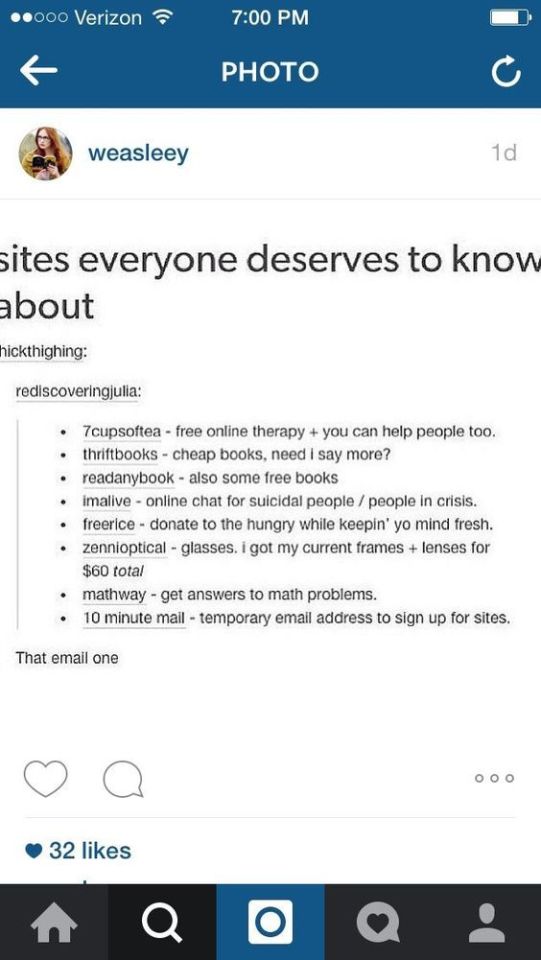
Ι've checked most of them and they are legit


Hey can anyone understand/ read my handwriting? It’s for my biotech report and I’m not sure in my profs will be able to understand it.




As a neurodivergent person who didn’t get much help with the sciences last year, I wanted to post this as review for others. Hope it helps!
Image ID:
First:
A periodic table which is brightly coloured with the colours of the rainbow. They are coloured in order of pink, pink-orange, yellow, light green, green, blue and purple. They are coloured to show the various kinds of elements (alkali metals, halogens, etc.)
Second:
A brightly coloured note which explains what groups and periods are. Groups = vertical, periods = horizontal. Groups tell how many valence electrons and element has, and periods show how many energy levels / shells they have.
Third: Another brightly coloured note which explains the rules of the periodic table. Metals get more reactive when you go down and to the left, while nonmetals get more reactive when you go up and to the right. An exception being stated as noble gases, due to how they are already stable. It also states the location and charge of protons, neutrons and electrons. The protons and neutrons are shown to be located in the nucleus while the electrons are shown to be outside. Protons have a positive charge- neutrons with a neutral, and electrons with a negative.
Fourth:
A green box showcases the locations of important text on an element. The element example is Oxygen. It also states how to find the amount of neutrons, protons and electrons. Protons and electrons are able to be found through the atomic number, while the neutrons need an equation. You must subtract the atomic mass - the atomic number to figure that out. The equation is 16-8=8. Therefore there are 8 Neutrons in oxygen.
ID END
I just made this acronym up and if anyone needs this for science to help memorize the periodic trends, here it is!
Atomic radius
Ionization energy
Reactivity
Electronegativity
Electron affinity
Almost all of the trends go up and to the right = stronger, with the atomic radius going down and to the left = stronger!
It has been almost two hours since i have started my homework and i would just like to ask how I'm meant to "explain sentence structure" can someone help?
This is late homework by the way and it's due today so i need help fast
THIS IS EXTREMELY URGENT SOMEONE HELP PLEASE
I think i figured it out but i probably did it wrong
Oh well, at least i don't all my homework even though it did take over 3 hours

I HAVE TO DO ALL OF THESE FRONT AND BACK BEFORE TUESDAY 👹