Chemiballs - Tumblr Posts
Meet the Chemiballs: The Metagaming Metalloids!
Meet the Chemiballs: The Metagaming Metalloids!

View On WordPress
How To Understand Resonance Structures using Cute Balls
Are you taking an org chem class and don't understand what "electron delocalization" is at all? Would cute country ball esque drawings make it easier to understand or at the very least make you fell a bit better about taking an org chem class and not understanding what "electron delocalization" is at all?
Link to article
Meet the Chemiballs; the Explosive Alkali Metals!

View On WordPress
Meet the Chemiballs: the Nuclear Nucleic Acids
Did you know that your DNA is made out of little cartoon balls with unhealthy relationship dynamics?


View On WordPress
Meet the Chemiballs: the Nuclear Nucleic Acids
Did you know that your DNA is made out of little cartoon balls with unhealthy relationship dynamics?


View On WordPress
Sorry if I don't answer asks right away, I don't always have time or am in the right headspace to be able to meet my standard of quality. Most things on my blogs are queued.
Also, I have a side blog called @ravings-of-a-mad-scientist-2 for reblogs, replies, off topic asks, and miscellaneous thingos so that the main blog doesn't get cluttered, FOLLOW IT NOW!
My new "main" blog is @erose-this-name, it's not at all like these other two blogs! (I also write psych horror scifi) FOLLOW ME, OR BE SUBSUMED INTO THE VALLEY OF FLESH!
Tags:
#scientists sitcom - funny skits involving -ologists, very funny, very popular
#hero x villain - I guess this tag is it's whole own thing, but I do them because I think it's kinda funny to make short skits parodying the heroes to lovers genre involving characters that are parodies of comic book and romance tropes.
#asks - I love getting asks!
#mad scientist - You see my blog's name, right? You know what website this is? What do you think is my stance on mad scientists?
#chemiballs - like countryballs, but elements and chemicals!
Meet the Chemiballs: Exposition and Cute Drawings of the Nonmetals
I love balls. Especially Countryballs, aka Polandball, a popular meme format where countries are embodied in this particular art style. Like Hetalia but for not-weebs. I mean, just look at r/countryball. These little blobs are so cute.
Lots of other "ball communities" have spawned from Countryballs, such as Ideologyballs, Planetballs, and LGBallT. One of the little-known ones is Chemiballs. At first, I thought I was the first one to have the idea as I doodled them in my notebook during chemistry class, but no. Someone beat me to it just a couple of years before me. (hecking go check out the subreddit it's a great small community https://www.reddit.com/r/chemiball/)
Anyways here's some nonmetal balls I drew a couple years ago, AREN'T THEY CUTE??






here's an old edutainment blog page I wrote about the these elements if you're interested
Meet the Chemiballs: the Postal Post Transition Metals
The post transition metals are pretty much what they say on the tin. No, these aren’t post-op transgender metals, they’re the metals that come after the transition metals on the periodic table!




Gallumball has a very low melting point (you can melt it with bodyheat), and molten gallumblob has similar properties to mercuryblob but isn't poisonous!

Tinball getting thanos snapped (tin metal likes to turn to dust when it gets too cold, a phenomenon known as tinpest)


I’ve thought long and hard about how to describe bismuth in a better way than the somewhat mad scientist Tom from Explosions&Fire. But I can’t, so I’m just gonna plagiarize quote him.
“[Bismuth] has seen a bit of a revival lately as a bit of a ‘relatable icon element’ because it forms rainbows all the time, doesn’t like to be straight, and is incredibly dense. Personally, I’ve always thought of bismuth as lead for people who fear death.” -Tom

here's an article I wrote years ago about the post transition metals (where the art is from)
Dumb and very esoteric chemiball comic about the mechanism of organic mercury poisoning

Selenium (Se) is a necessary micronutrient. It’s involved in the amino acid selenocysteine (Sec), which is like cysteine (Cys) but instead of sulfur, it’s selenium. Selenocysteine is involved in selenoenzymes, which are important antioxidants.
Oxidants are highly reactive oxygen radicals that are sometimes accidentally made by the mitochondria. They really like to oxidize and destroy things like proteins, fats, and DNA. As it turns out, we need those things to not be destroyed.
Antioxidants are important because they’re useless and don’t do anything important other than be tempting targets for oxidants. They take the hit so you don’t have to. (though knowing biochemistry, I’m sure that most of them actually do everything, because it would just make too much sense otherwise.)
Organic mercury poisoning is bad because it permanently inhibits your selenoenzymes. This leaves the cell vulnerable to oxidation which is particularly bad for your brain cells.
In this case, methyl mercury (Hg-CH3) has previously bonded to the sulfur in Cysteineball and has now come near the selenocysteine ball.
Fun fact: the covalent binding affinities between mercury and selenium are about A MILLION times greater than with sulfur. I’m not exaggerating, it’s literally cited as ONE MILLION TIMES GREATER. So yeah, Mercuryblob immediately jumps to seleniumball and basically never lets go.
Sources: Ralston, N. & Azenkeng, Alexander & Raymond, Laura. (2012). Mercury-Dependent Inhibition of Selenoenzymes and Mercury Toxicity. 10.1007/978-1-4614-2383-6_5. https://www.researchgate.net/publication/278702735_Mercury-Dependent_Inhibition_of_Selenoenzymes_and_Mercury_Toxicity
Ralston NVC, Raymond LJ. Mercury’s neurotoxicity is characterized by its disruption of selenium biochemistry. Biochim Biophys Acta Gen Subj. 2018 Nov;1862(11):2405-2416. doi: 10.1016/j.bbagen.2018.05.009. Epub 2018 May 9. PMID: 29753115.
Meet the Chemiballs: Halogen Shenanigans
“Halogen” is a collective name for the elements that fall under fluorine in the periodic table. They all have the naming convention of ending in “-ine”, so you always know when something is a halogen. Though the same naming convention is used for a lot of random chemicals, like cadaverine or quinine, so it’s not a perfect system.
They're pretty scary chemicalballs 'cause they really like electronballs. Like, they really heckin' like 'em. They'll sell your own mother just to get a sweet sweet taste of that electronegativity.

POV you are being mugged by Flourineball. Hand over your electrons.




Astatineball is horrified as Tennessineball fades away after giving its final message. Astatineball knows it has a 50% chance of being doomed to the same fate in 8.1 hours or so (both elements have very short half-lives)

for more info i guess
Meet the Chemiballs: Some* of the Transition Metals
More Chemiballs! This time we’re looking at the Transition Metals. The Transition Metals are that flat bit in the middle of the periodic table. It’s defined as being all the elements that have a “d” orbital as their valence shell, which I’m sure there’s a very immature joke to be made of. But I’m far too mature and adult to stoop that low. (hehe, Transition metals)
* bruh look, im not gonna draw all the transition metals. have you SEEN a periodic table recently? do you have any idea how many transition metals there are? like, 34 or something. and 90% of them would just be varying shades of gray. i just drew a few cool ones







here's an article I wrote years ago about the transition metals (where the art is from)
Comic about Mercuryblob AMALGAMATING Aluminumball

Meet the Chemiballs; the Explosive Alkali Metals!
The alkali metals are the first group on the periodic table. Their whole thing is; they have some valence electrons, but would rather that not be the case. So they really like to pair up with electronegative elements, like oxygenball, who can take their electrons. Thus allowing the alkali metals to dissolve into solution as cute cation cats (deadbead dads).







A old funny blog article with more EDUTAINING POWER regarding alkalimetalballs.
Sodium Hydroxide + Hydrochloric Acid

The Hydrogenball ships them.
Meet the Chemiballs; Earthbreaking Alkaline Earth Metals
The Alkaline Earth Metals are a bit like a diet version of the alkali metals. Unlike the alkali metals who have one valence electron that they really want to get rid of, alkaline earth metals have two that they sorta dislike having. They’re still very energetically reactive, often bursting into flames during chemical reactions. But they’re less likely to outright explode as sodiumball or potassiumball are.







Here's an old article with informative information about Alkaline Earth Metal balls but that's also meant to be funny i guess
Meet the Chemiballs; the Noble Noble Gases
The noble gases were all (mostly) discovered by a Scottish man named Sir William Ramsay (Though, back in his day, it was more common to call them “rare gases”). He made the convention of ending all their names with -on, so you always know when something is a noble gas. (Unless it’s helium which is a noble gas but follows the metal naming convention, [or iron which ends in -on but is a metal. {Also, scientists seem to really like giving things -on names, like prion, codon, electron, etc. I will admit, it does sound cool. }]) So it’s not a perfect system.
The name “noble gasses” is a bit of an early 1900s joke. See, the noble gasses are too lazy to do anything and don’t like bonding with lesser peasant elements. The nobility is also lazy and don’t like associating with peasants. Of course, we live in an enlightened post-WWI world and no longer recognize barbaric concepts such as hieratical rule and rigid class structures. Also, the Queen is dead. But this is basically the equivalent of naming them “trust fund gasses”. The more things change, the more they stay the same.








Meet the Chemiballs: The Metagaming Metalloids!
So you know metals? They’re metallic, conductive, and usually form positively charged ions? And you know nonmetals? They’re not metallic, insulators, and make negatively charged ions. So yeah, what do you call stuff that’s like, not one of those two things? Y’know, semiconductors and shit.
Metals are only metallic because they’re bad at holding on to their valence electrons, and elements tend to get worse at that the closer they are to the bottom left of the periodic table. Because physics.
But there’s a stair-shaped boundary line between the two where it’s really hard to decide if they’re one or the other so we sort of just gave up and called them metalloids.








READ MY OLD BLOG IT HAS JOKES AND FUN FACTS ABOUT ELEMENTS AAAAHHH
Meet the Chemiballs; the Active Actinides
The actinides are famous for being very radioactive and generally not safe to be around. They contain a few elements that are very interesting and important. But also a whole lot of very obscure and boring elements (I didn't draw those).
They are one of the two rows awkwardly separated underneath the periodic table, the other being the Lanthanides (which I have not drawn any of yet, sorry).
Both of these make up the f orbital block (which is to say their valence electrons orbit in a very strange shape). The f block technically should be placed between the transition metals and alkali earth metals. But isn’t because that would make the table far too long to fit on the walls of chemistry classrooms.








Meet the Chemiballs; The Particularly Spooky Subatomic Particles!






![W Bosonballs and Z Bosonball being overfed too many tacos by very hospitable Higgs Bosonball (The higgs boson gives the W and Z bosons MASS, which by extension give all [or most?] other matter mass)](https://64.media.tumblr.com/68a054e435301993ee14259100949ff9/69a482e27961dc88-df/s500x750/aa4a5963cc7039ee2aacb4d6000cd1a6a1e63a23.png)

Read this old blog post of mine where I, a biomajor, tried and failed miserably to understand quantum physics :3
How To Understand Resonance Structures using Cute Balls
There comes a time in every man’s (or woman/nonbinary/other/etc)'s life that they have to learn about resonance structures and delocalized electrons.
Or probably not. I don’t think most people know what it is. But you’re going to!
Right now.
No, you don’t have a choice in the matter.

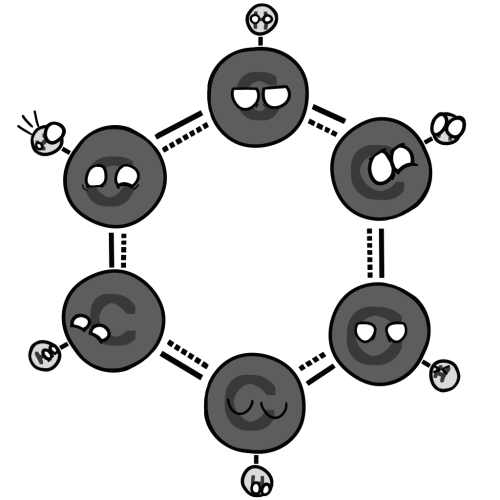
![Pentadienyl cation with some very insecure carboCATionballs
(positively charged atoms/molecules are called CATions. Carbonball REALLY doesn't like being a +1 cation, but can tolerate being one third of a furry.[veru scientific{physics isn't voodoo, no siree}])](https://64.media.tumblr.com/6435aa88dc4bbbdbba289ebaa924bbeb/1d39ed985ba14fc1-08/s500x750/7cfb61082c131c829ca0c1351535b31997c34e10.png)

Get all that? Understand now? Of course not, no one does. READ THIS!

ARTICLE I MADE YEARS AGO FOR MORE IN-DEPTH AND FUNNY EXPLANATION!